
Chemistry, 13.12.2021 07:10, heybrothwrlogan
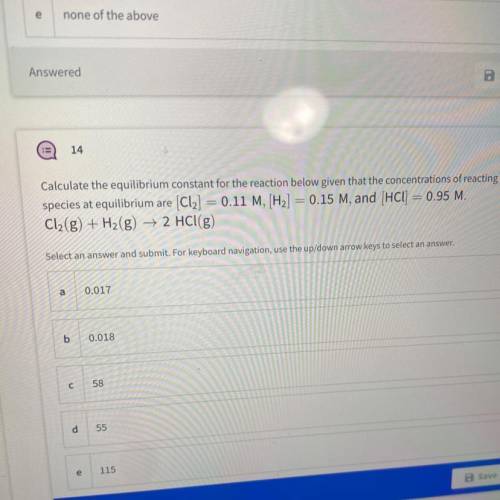
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting
species at equilibrium are (Cl2] = 0.11 M, [H2] = 0.15 M, and (HCl) = 0.95 M.
Cl2(g) + H2(g) → 2 HCl(g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Do you know the correct answer?
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting...
Questions in other subjects:




Biology, 31.01.2020 17:57


Physics, 31.01.2020 17:57




Mathematics, 31.01.2020 17:57






