-
-
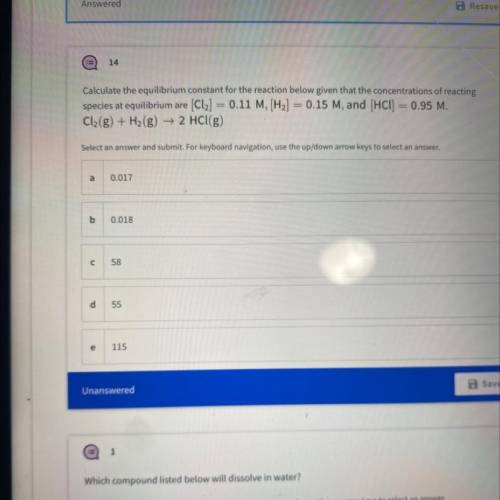
Calculate the equilibrium constant for the reaction below given that the concentrations...

Chemistry, 13.12.2021 06:40, ninigilford
-
-
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting
species at equilibrium are [Cl2] = 0.11 M, [H2] = 0.15 M, and (HCl) = 0.95 M.
Cl2(g) + H2(g) → 2 HCl(g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, cicimarie2018
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 06:30, themajesty9898
The minerals found in bones are deposited by living cells called
Answers: 1

Do you know the correct answer?
Questions in other subjects:

Physics, 23.10.2020 16:40

English, 23.10.2020 16:40

Mathematics, 23.10.2020 16:40

History, 23.10.2020 16:40

Mathematics, 23.10.2020 16:40





Mathematics, 23.10.2020 16:40







