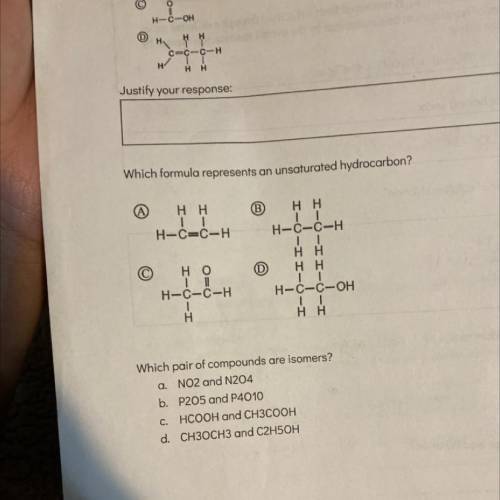
Which formula represents an unsaturated hydrocarbon?
(А)
Hн
H-C=C-H
B) Hн
...

Chemistry, 12.12.2021 23:00, jessiebotello7209
Which formula represents an unsaturated hydrocarbon?
(А)
Hн
H-C=C-H
B) Hн
H-C-C-H
ТІ
Hн
Hн
| |
н-с-с-ОН
но
н-с-с-Н
т
Н
- ..
Hн.
Which pair of compounds are isomers?
a. No2 and N204
b. P205 and P4010
C. HCOOH and CH3COOH
d. CH3OCH3 and C2H5OH

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
Do you know the correct answer?
Questions in other subjects:


English, 05.07.2019 10:10








Health, 05.07.2019 10:10






