
Chemistry, 10.12.2021 21:30, dessyrob05
In the system 2A + B = 2C K = 2.25 x 10^5, the initial concentrations are A = 2.35 M, B = 3.25 M, and C = 0 M. Calculate their equilibrium concentrations.
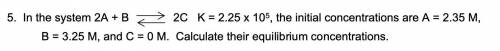

Answers: 3
Other questions on the subject: Chemistry




Do you know the correct answer?
In the system 2A + B = 2C K = 2.25 x 10^5, the initial concentrations are A = 2.35 M,
B = 3.25 M,...
Questions in other subjects:

Biology, 23.09.2019 19:10






Health, 23.09.2019 19:10









