
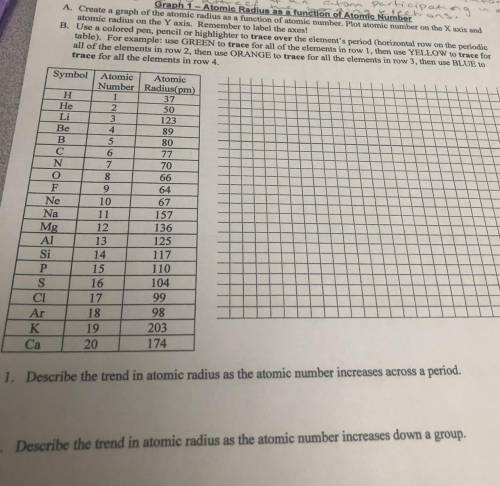
Graph 1 - Atomic Radius as a function of Atomic Number
A. Create a graph of the atomic radius as a function of atomic number. Plot atomic number on the X axis and
atomic radius on the Y axis. Remember to label the axes!
B. Use a colored pen, pencil or highlighter to trace over the element's period (horizontal row on the periodic
table). For example: use GREEN to trace for all of the elements in row 1, then use YELLOW to trace for
all of the elements in row 2, then use ORANGE to trace for all the elements in row 3, then use BLUE to
trace for all the elements in row 4.
Symbol
H
He
Li
Be
B
С
N
O
F
Ne
Na
Mg
Al
Si
P
S
СІ
Ar
K
Ca
Atomic Atomic
Number Radius(pm)
1
37
2
50
3
123
4
89
5
80
6
77
7
70
8
66
9
64
10
67
11
157
12
136
13
125
14
117
15
110
16
104
17
99
18
98
19
203
20
174
1. Describe the trend in atomic radius as the atomic number increases across a period.
2. Describe the trend in atomic radius as the atomic number increases down a group.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, milkshakegrande101
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 03:30, babygirl1780
Nanotechnology, the field of trying to build ultrasmall structures one atom at a time, has progressed in recent years. one potential application of nanotechnology is the construction of artificial cells. the simplest cells would probably mimic red blood cells, the body's oxygen transporters. for example, nanocontainers, perhaps constructed of carbon, could be pumped full of oxygen and injected into a person's bloodstream. if the person needed additional oxygen-due to a heart attack perhaps, or for the purpose of space travel-these containers could slowly release oxygen into the blood, allowing tissues that would otherwise die to remain alive. suppose that the nanocontainers were cubic and had an edge length of 24 nanometers. part a part complete what is the volume of one nanocontainer? (ignore the thickness of the nanocontainer's wall.) express your answer using two significant figures. v v = 1.4ă—10â’20 l previous answers correct significant figures feedback: your answer 1.3824â‹…10â’20 = 1.382ă—10â’20 l was either rounded differently or used a different number of significant figures than required for this part. if you need this result for any later calculation in this item, keep all the digits and round as the final step before submitting your answer. part b suppose that each nanocontainer could contain pure oxygen pressurized to a density of 81 g/l . how many grams of oxygen could be contained by each nanocontainer?
Answers: 3

Chemistry, 23.06.2019 03:10, 3jazybraxy
Which is true according to the law of conservation of energy
Answers: 1
Do you know the correct answer?
Graph 1 - Atomic Radius as a function of Atomic Number
A. Create a graph of the atomic radius as a...
Questions in other subjects:

Mathematics, 12.01.2021 22:40

Mathematics, 12.01.2021 22:40

Social Studies, 12.01.2021 22:40



Mathematics, 12.01.2021 22:40

Mathematics, 12.01.2021 22:40

Mathematics, 12.01.2021 22:40


Mathematics, 12.01.2021 22:40






