
Chemistry, 10.12.2021 02:00, shadestephen25
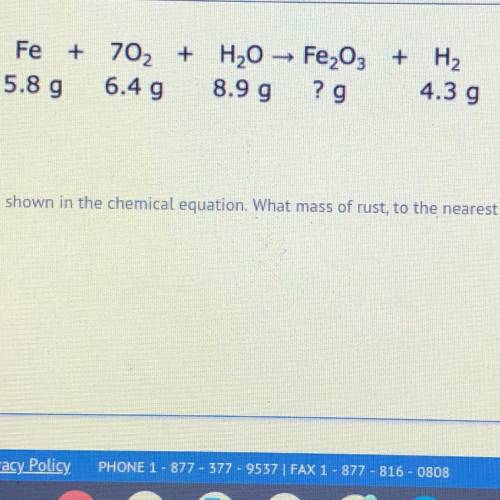
iron reacts with oxygen and water to create rust and hydrogen gas as shown in the chemical equation. what mass of rust, to the nearest hundredth gram, is produced in this reaction?

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 06:00, BigGirlsTheBest
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2
Do you know the correct answer?
iron reacts with oxygen and water to create rust and hydrogen gas as shown in the chemical equation....
Questions in other subjects:




Mathematics, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00


Mathematics, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00







