
Consider the equations below.
4 equations. 1, Upper C Upper H Subscript 4 Baseline (g) right arrow Upper C (s) + 2 Upper H Subscript 2 Baseline (g) Delta H Subscript 1 Baseline = 74.6 kilojoules. 2, Upper C (s) + 2 Upper C l Subscript 2 Baseline (g) right arrow Upper C Upper Cl Subscript 4 Baseline (g) Delta H Subscript 2 Baseline = negative 95.7 kilojoules. 3, 2 Upper H Subscript 2 Baseline (g) + 2 Upper C l Subscript 2 Baseline (g) right arrow 4 Upper H Upper Cl (g) delta H Subscript 3 Baseline = negative 284.6 kilojoules. 4, Upper C Upper H Subscript 4 Baseline (g) + 4 Upper C l Subscript 2 Baselines (g) right arrow Upper C Upper C l Subscript 4 Baseline (g) + 4 Upper H Upper C L (g) Delta H 4 = negative 205.7 kilojoules.
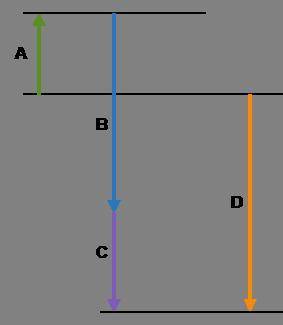
Complete the following based on the diagram.
Arrow A:
Arrow B:
Arrow C:
Arrow D:

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 02:30, jrjimenez
At 40 âc the solution has at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. g of kno3 per 100 g of water and it can contain up to at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. g of kno3 per 100 g of water. at 0 âc the solubility is ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. kno3 per 100 g of water, so ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. gkno3 per 100 g of water will precipitate out of solution. a kno3 solution containing 55 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 2

Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Do you know the correct answer?
Consider the equations below.
4 equations. 1, Upper C Upper H Subscript 4 Baseline (g) right arrow...
Questions in other subjects:





History, 08.10.2019 03:40

Mathematics, 08.10.2019 03:40

History, 08.10.2019 03:40

History, 08.10.2019 03:40


History, 08.10.2019 03:40






