
Chemistry, 09.12.2021 06:20, QueenNerdy889
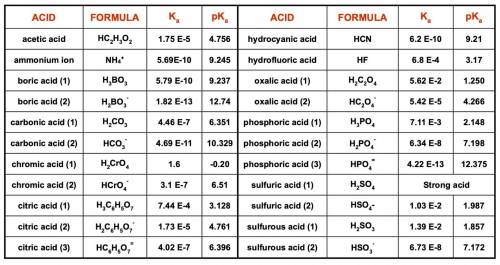
1. List the species present at equilibrium in a solution with the following
composition:
NH4Cl = 0.0200 mol/L NaOH = 0.0430 mol/L
H2SO4 = 0.0150 mol/L NaNO3 = 0.0100 mol/L
2. Write the n equations for n unknowns describing the equilibrium composition of
this system.
3. Make a spreadsheet and use Excel’s Solver function to determine the equilibrium
pH and concentrations of all species in this solution.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2


Chemistry, 22.06.2019 21:30, sullivanjakob
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
Do you know the correct answer?
1. List the species present at equilibrium in a solution with the following
composition:
Questions in other subjects:

Physics, 15.10.2019 11:20

Mathematics, 15.10.2019 11:20


History, 15.10.2019 11:20

Mathematics, 15.10.2019 11:20










