
Chemistry, 09.12.2021 05:50, natnerd4671
For parts of the free-response question that require calculations, clearly show the method used and the steps involved in arriving at your answers. You must show your work to receive credit for your answer. Examples and equations may be included in your answers where appropriate.
2NO2(g)+F2(g)→NO2F(g)
ΔH∘rxn=−284kJ/molrxn
NO2(g) and F2(g) can react to produce NO2F(g), as represented above. A proposed mechanism for the reaction has two elementary steps, as shown below.
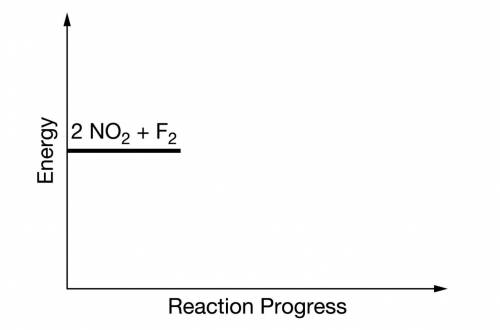
(b) On the incomplete reaction energy diagram below, draw a curve that shows the following two details.
The relative activation energies of the two elementary steps
The enthalpy change of the overall reaction

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1



Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2
Do you know the correct answer?
For parts of the free-response question that require calculations, clearly show the method used and...
Questions in other subjects:

History, 03.06.2021 08:40

English, 03.06.2021 08:40


Mathematics, 03.06.2021 08:40


English, 03.06.2021 08:40

Geography, 03.06.2021 08:40


History, 03.06.2021 08:40

English, 03.06.2021 08:40






