NEED HELP ASAP PLZZ PLATO
Temperature and Reaction Rate
In this task, you will determine how...

Chemistry, 07.12.2021 04:20, a897coleman
NEED HELP ASAP PLZZ PLATO
Temperature and Reaction Rate
In this task, you will determine how the temperatures of the reactants affect how quickly a chemical reaction takes place. To do so, you’ll measure the reaction rate indirectly by timing how long the reaction takes to complete. Before you begin this task, read these lab safety guidelines. If you’re using a graduated cylinder and an electronic balance, watch these videos about measuring volume and measuring mass.
Estimated time to complete: 90 minutes
If you’ve purchased an Edmentum lab kit, remove the electronic balance, graduated cylinder, thermometer, and safety goggles. These materials are italicized in the following equipment list. Then gather any additional items shown in the list. If you’re not using an Edmentum lab kit, alternatives are suggested in parentheses.
You’ll need the following materials:
graduated cylinder (may also use a 1-tablespoon measure)
electronic balance, precise to at least 0.1 gram, with weighing boat (may also use a
1
4
-teaspoon measure)
thermometer, readable from 0°C to 100°C (32°F to 212°F)
safety goggles
3
4
cup (180 milliliters) vinegar
1 tablespoon (18 grams) baking soda
water
stove, hot plate, or microwave oven (for heating water)
container appropriate for heating water
beaker, flask, or cup
spoon or stirring rod
stopwatch
Stay safe! Be careful while heating water or when handling hot water to avoid burns. Be sure to wear safety goggles while performing the experiment.
Part A
In this task, you’ll observe a chemical reaction between baking soda and vinegar. How do you think the temperature of the reactants and the rate of reaction are related? Write a hypothesis to predict the relationship between the two parameters.
Part B
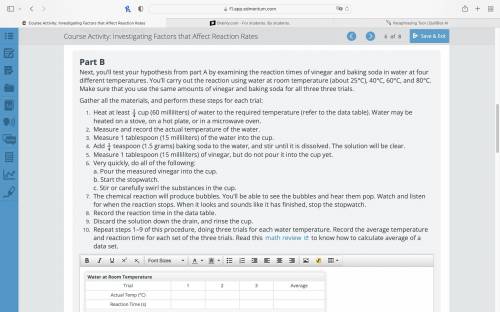
Next, you’ll test your hypothesis from part A by examining the reaction times of vinegar and baking soda in water at four different temperatures. You’ll carry out the reaction using water at room temperature (about 25°C), 40°C, 60°C, and 80°C. Make sure that you use the same amounts of vinegar and baking soda for all three three trials.
Gather all the materials, and perform these steps for each trial:
Heat at least
1
4
cup (60 milliliters) of water to the required temperature (refer to the data table). Water may be heated on a stove, on a hot plate, or in a microwave oven.
Measure and record the actual temperature of the water.
Measure 1 tablespoon (15 milliliters) of the water into the cup.
Add
1
4
teaspoon (1.5 grams) baking soda to the water, and stir until it is dissolved. The solution will be clear.
Measure 1 tablespoon (15 milliliters) of vinegar, but do not pour it into the cup yet.
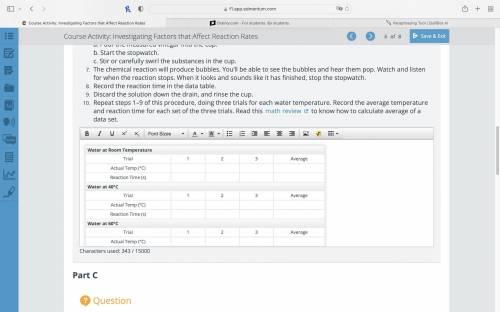
Very quickly, do all of the following:
a. Pour the measured vinegar into the cup.
b. Start the stopwatch.
c. Stir or carefully swirl the substances in the cup.
The chemical reaction will produce bubbles. You’ll be able to see the bubbles and hear them pop. Watch and listen for when the reaction stops. When it looks and sounds like it has finished, stop the stopwatch.
Record the reaction time in the data table.
Discard the solution down the drain, and rinse the cup.
Repeat steps 1–9 of this procedure, doing three trials for each water temperature. Record the average temperature and reaction time for each set of the three trials. Read this math review to know how to calculate average of a data set.
Part C
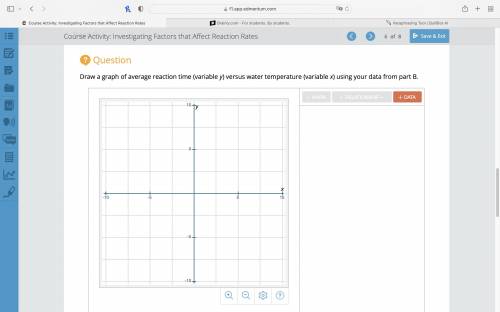
Question
Draw a graph of average reaction time (variable y) versus water temperature (variable x) using your data from part B.
Part D
Explain how the reaction time and the reaction rate compare to the temperature of the water and the reactants. Revisit your hypothesis from part A, and explain how it compares to your experimental results.
Part E
Study the trend of the graph from part C. What would the reaction time be (in seconds) if the water were cooled to 5°C?
Part F
Why is it a good idea to perform three trials at each temperature? How would you modify the experiment to produce more reliable results?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, lejeanjamespete1
Heat flow inside earth activity i chose chemistry cause there is no science alert! the newspaper has been hacked, and all of the headlines have been changed to reflect old legends. it is your task to make sure they are corrected. choose one of the newspaper headlines. you will rewrite the headline and a one-to-two-paragraph article to make the news scientifically correct. be sure to include the following in your corrected newspaper article: new title reflecting correct information detailed information about the processes and tectonic plate interactions that are causing the geological event to occur real-world example of where to find the geological event on earth (picture and location) also review the grading rubric before you begin.
Answers: 1

Chemistry, 22.06.2019 02:30, Countryqueen525
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Biology, 04.05.2020 23:16


Mathematics, 04.05.2020 23:16

Social Studies, 04.05.2020 23:16

Mathematics, 04.05.2020 23:16



Chemistry, 04.05.2020 23:16


History, 04.05.2020 23:16






