
Chemistry, 02.12.2021 21:30, maciemarklin3032
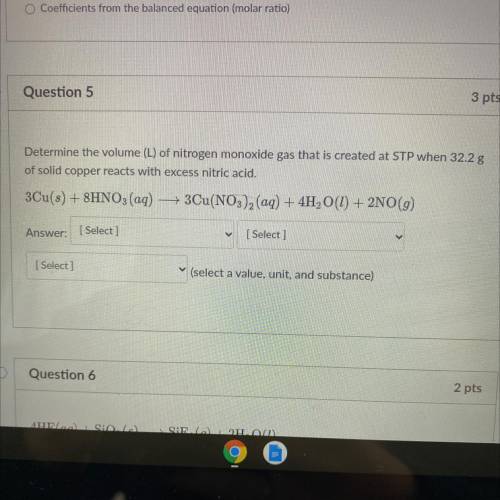
Determine the volume (L) of nitrogen monoxide gas that is created at STP when 32.2 g
of solid copper reacts with excess nitric acid.
3Cu(s) + 8HNO3(aq) — 3Cu(NO3)2 (aq) + 4H2O(1) + 2NO(g)

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1
Do you know the correct answer?
Determine the volume (L) of nitrogen monoxide gas that is created at STP when 32.2 g
of solid copp...
Questions in other subjects:



Mathematics, 12.11.2019 00:31












