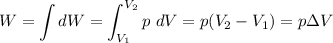Chemistry, 01.12.2021 23:50, lannor6586
How to calculate work done with change in volume and pressure.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1
Do you know the correct answer?
How to calculate work done with change in volume and pressure....
Questions in other subjects:


Physics, 02.07.2019 22:30

Mathematics, 02.07.2019 22:30



History, 02.07.2019 22:30


Mathematics, 02.07.2019 22:30

Mathematics, 02.07.2019 22:30