The Mole
7
Acellus
Now that you know the scaling factor, simply
multiply each su...

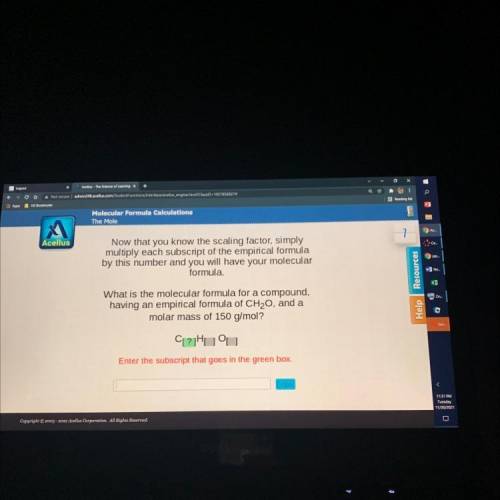
The Mole
7
Acellus
Now that you know the scaling factor, simply
multiply each subscript of the empirical formula
by this number and you will have your molecular
formula.
on Resources
What is the molecular formula for a compound,
having an empirical formula of CH20, and a
molar mass of 150 g/mol?
CL?Huu
Enter the subscript that goes in the green box.
Enter

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 20:00, 20calzoy
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
Do you know the correct answer?
Questions in other subjects:



History, 15.10.2020 05:01


Chemistry, 15.10.2020 05:01




History, 15.10.2020 05:01

Mathematics, 15.10.2020 05:01







