Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:00, jlegrand9098
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 13:00, brycehelmke60811
Using the periodic table complete the table to describe each atom type in your answers
Answers: 1

Do you know the correct answer?
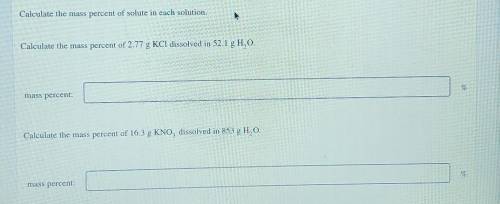
Calculate the mass percent of solute in each solution. Calculate the mass percent of 2.77 g KCl diss...
Questions in other subjects:


Physics, 19.07.2019 10:30