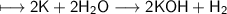Chemistry, 28.11.2021 09:50, sterlingrobinson35
When potassium metal is placed in water, a large amount of energy is released as potassium hydroxide and hydrogen gas are produced in the reaction 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g). Your lab partner says this is a redox reaction and a combustion reaction. Do you agree? Defend your answer by explaining whether or not it meets the requirements of each type of reaction.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 23.06.2019 00:40, joe7977
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 02:30, kieante01
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
Do you know the correct answer?
When potassium metal is placed in water, a large amount of energy is released as potassium hydroxide...
Questions in other subjects:


Mathematics, 14.05.2021 01:00



Mathematics, 14.05.2021 01:10

Social Studies, 14.05.2021 01:10



Mathematics, 14.05.2021 01:10

Spanish, 14.05.2021 01:10