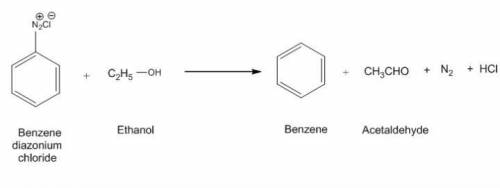
Conversion of ethanol to benzene (class 11th)
Ch3Ch2OH ——- C6H6(benzene)...

Chemistry, 25.11.2021 14:00, MayFlowers
Conversion of ethanol to benzene (class 11th)
Ch3Ch2OH ——- C6H6(benzene)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, hannah5143
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 21.06.2019 18:00, mykalwashington
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 01:30, arodavoarodavo
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 14.12.2020 19:50




Physics, 14.12.2020 19:50




Mathematics, 14.12.2020 19:50