
Chemistry, 23.11.2021 23:00, runaway173
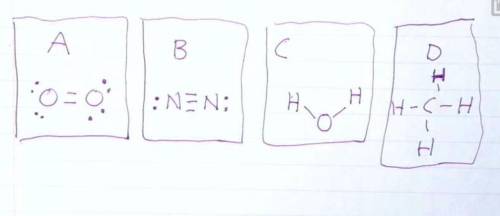
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing on this test and select the answer that best describes which drawing is wrong and why.
Question 6 options:
A: O2 Is wrong because it shows the electrons at a 45 degree angle to the Oxygen atoms.
B: N2 is wrong because it shows a triple bond.
C: H2O is wrong because it is missing 4 valence electrons.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Do you know the correct answer?
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing o...
Questions in other subjects:


History, 18.06.2020 10:57

Mathematics, 18.06.2020 10:57

Mathematics, 18.06.2020 10:57

History, 18.06.2020 10:57

Chemistry, 18.06.2020 10:57

Physics, 18.06.2020 10:57

Mathematics, 18.06.2020 10:57

History, 18.06.2020 10:57

Mathematics, 18.06.2020 10:57







