
Chemistry, 10.11.2021 20:10, theatergeek005
Topics: Fundamentals of electrochemistry; Electrochemical cells
Use general chemistry rules for significant figures.
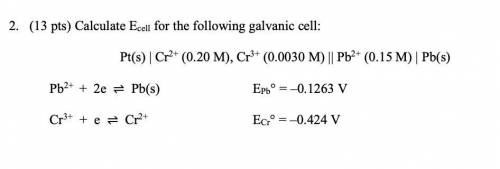
2. (13 pts) Calculate Ecell for the following galvanic cell:
Pt(s) | Cr2+ (0.20 M), Cr3+ (0.0030 M) || Pb2+ (0.15 M) | Pb(s)
Pb2+ + 2e ⇌ Pb(s) EPb° = –0.1263 V
Cr3+ + e ⇌ Cr2+ ECr° = –0.424 V

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 23.06.2019 04:00, Bassoonist
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
Do you know the correct answer?
Topics: Fundamentals of electrochemistry; Electrochemical cells
Use general chemistry rules for si...
Questions in other subjects:


Mathematics, 17.12.2020 17:50





History, 17.12.2020 17:50

Computers and Technology, 17.12.2020 17:50

Mathematics, 17.12.2020 17:50






