
Chemistry, 10.11.2021 19:20, daelinrobinson
Use general chemistry rules for significant figures.
1. (12 pts) A cell is constructed to reduce the Ag+ in AgCl(s) to Ag(s) at the cathode when
current is passed through the cell. At the anode, Cu(s) is oxidized to Cu2+.
If current of 0.500 A is passed through the cell for 101 minutes, calculate the mass of
Cu(s) dissolved and mass of Ag(s) deposited. Half-cell reactions:
Cathode: AgCl(s) + e ⇌ Ag(s) + Cl–
Anode: Cu(s) ⇌ Ce2+ + 2e
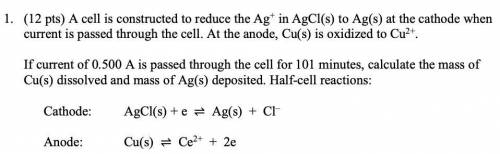

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Do you know the correct answer?
Use general chemistry rules for significant figures.
1. (12 pts) A cell is constructed to reduce t...
Questions in other subjects:

Mathematics, 30.11.2021 21:00




Spanish, 30.11.2021 21:00




Mathematics, 30.11.2021 21:00

Mathematics, 30.11.2021 21:00






