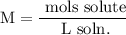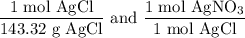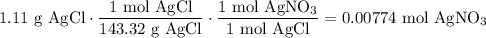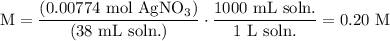Chemistry, 30.10.2021 19:00, jchavez0790
HELPPP PLS IM DESPRATE MAJOR POINTS NEED ASAP
We add excess NaCl solution (58.44 g/mol) to 38 mL of a solution of silver nitrate (AgNO3 169.88 g/mol), to form insoluble solid AgCl. When it has been dried and weighed, the mass of AgCl (143.32 g/mol) is found to be 1.11 grams.
What is the molarity of the original AgNO3 solution? The formula weight of NaNO3 is 85.00 g/mol.
Answer in units of M.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, minstcordell4115
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3


Chemistry, 22.06.2019 00:00, ashleyjaslin
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1
Do you know the correct answer?
HELPPP PLS IM DESPRATE MAJOR POINTS NEED ASAP
We add excess NaCl solution (58.44 g/mol) to 38 mL o...
Questions in other subjects:

Mathematics, 22.09.2021 14:00


Arts, 22.09.2021 14:00


Mathematics, 22.09.2021 14:00




Chemistry, 22.09.2021 14:00