A 50g block of aluminum at -100°C is submerged in 30g of water at 20°C.
Cwater = 4.184 J/gºC
...

Chemistry, 26.10.2021 19:30, lazavionadams81
A 50g block of aluminum at -100°C is submerged in 30g of water at 20°C.
Cwater = 4.184 J/gºC
AH tus water = 334 J/g
Cu = 0.89 J/gºC
What is the final temperature of both substances?
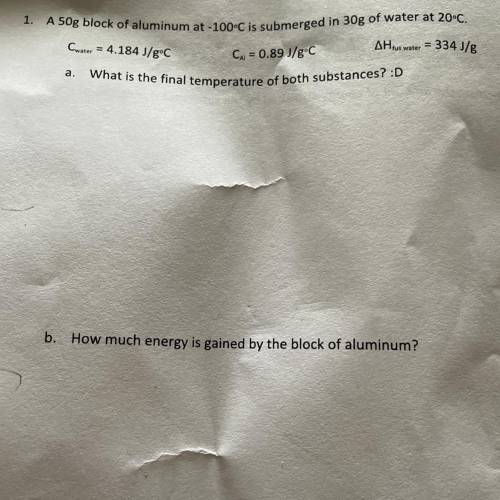

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Biology, 03.03.2021 03:10




English, 03.03.2021 03:10

Physics, 03.03.2021 03:10






