Chemistry, 25.10.2021 18:00, angelinaavila06
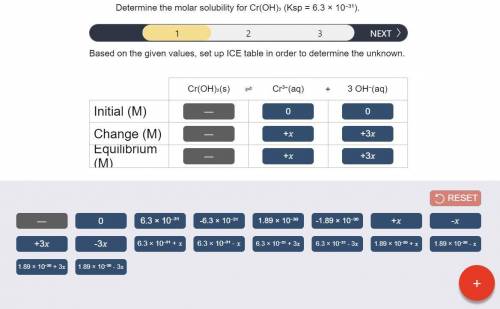
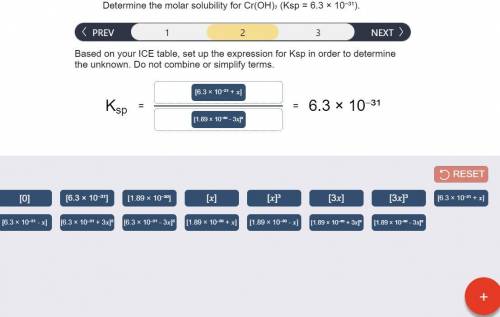
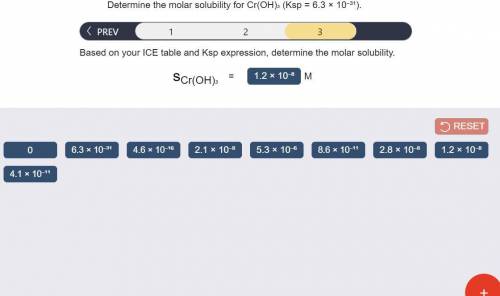
Determine the molar solubility for Cr(OH)3 Ksp = 6.3x10^-31 .
a) Set up the ICE table Cr(OH)3 (s) <-> Cr^3+ (aq) + 3OH^- (aq)
b) Ksp expression
c) Determine molar solubility

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
Do you know the correct answer?
Determine the molar solubility for Cr(OH)3 Ksp = 6.3x10^-31 .
a) Set up the ICE table Cr(OH)3 (s)...
Questions in other subjects:

Spanish, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

Chemistry, 10.09.2020 02:01


Mathematics, 10.09.2020 02:01

English, 10.09.2020 02:01


Mathematics, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01