Mit
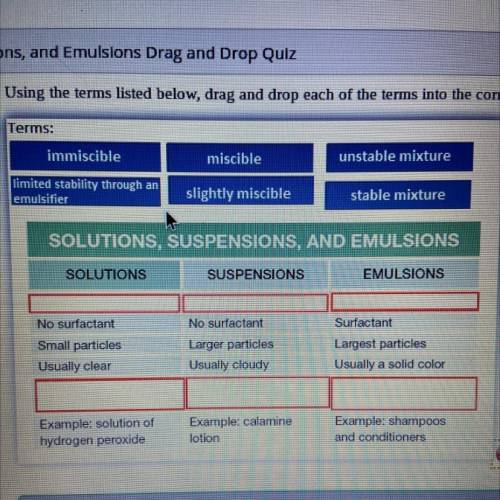
Terms:
immiscible
miscible
unstable mixture
pts (0.0%)
limited s...

Mit
Terms:
immiscible
miscible
unstable mixture
pts (0.0%)
limited stability through an
emulsifier
slightly miscible
stable mixture
at 7:03 PM
SOLUTIONS, SUSPENSIONS, AND EMULSIONS
SOLUTIONS
SUSPENSIONS
EMULSIONS
No surfactant
Small particles
Usually clear
No surfactant
Larger particles
Usually cloudy
Surfactant
Largest particles
Usually a solid color
Example: solution of
hydrogen peroxide
Example: calamine
lotion
Example: shampoos
and conditioners
***
Feedback

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Biology, 08.03.2021 23:40



Mathematics, 08.03.2021 23:40

Health, 08.03.2021 23:40


Mathematics, 08.03.2021 23:40









