
Chemistry, 25.10.2021 01:00, ahmedeldyame
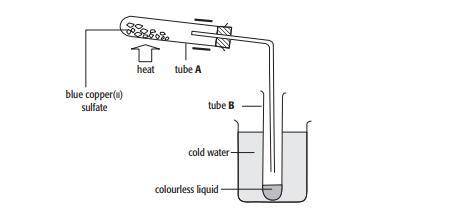
When 2.5 g of blue copper(ii) sulfate crystals were heated, 1.6 g of a white solid were left in tube A
a. Calculate the mass of water driven off in the experiment.
b. Calculate the percentage of water by mass driven off .

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
Do you know the correct answer?
When 2.5 g of blue copper(ii) sulfate crystals were heated, 1.6 g of a white solid were left in tube...
Questions in other subjects:

Mathematics, 16.04.2020 21:22


History, 16.04.2020 21:22



Mathematics, 16.04.2020 21:22


Chemistry, 16.04.2020 21:22








