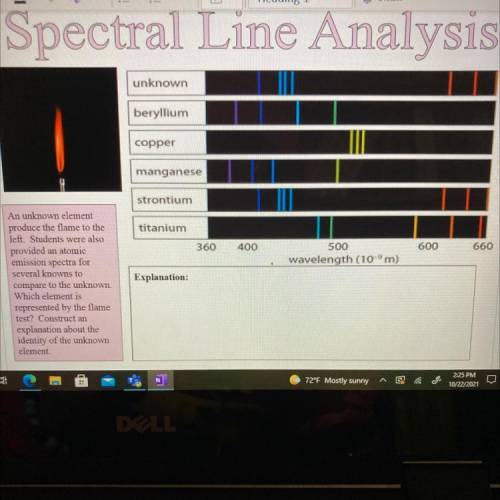
An unknown element
produce the flame to the
left. Students were also
provided an atomi...

Chemistry, 24.10.2021 08:20, jennelledenise
An unknown element
produce the flame to the
left. Students were also
provided an atomic
emission spectra for
several knowns to
compare to the unknown.
Which element is
represented by the flame
test? Construct an
explanation about the
identity of the unknown
element.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1


Do you know the correct answer?
Questions in other subjects:

Chemistry, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

Mathematics, 17.09.2020 22:01

History, 17.09.2020 23:01

Mathematics, 17.09.2020 23:01

Mathematics, 17.09.2020 23:01

Mathematics, 17.09.2020 23:01






