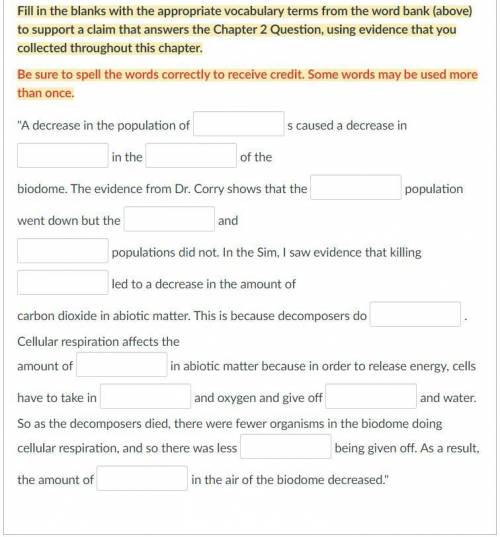
I DESPARATELY NEED HELP PLS
...

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:10, ArielA13
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3

Chemistry, 23.06.2019 16:00, jakebuttone
Afuel has 30.43% nitrogen and 69.57% oxygen. find the molecular formula of the compound if it has a mass of 92 grams per mole a. no b. n2o4 c. no2 d. n4o8
Answers: 1


Chemistry, 24.06.2019 06:50, AJSkullcrusher
A. circle the following formulas that represent ionic compounds. put a box around those that are covalent/molecular. no2 nacl bacl2 ch4 pbso4 h2o2 fe2o3 (nh4)2co3 ph3 2. know how to write the names and formulas for ionic compounds, covalent compounds, acids and bases. a. write the names for the following compounds: i. s4o6 iii. koh v. mg(cn)2 ii. fe3(po4)2 iv. co b. write the formulas for the following compounds: i. silicon dioxide iii. potassium fluoride v. cesium hypochlorite ii. lithium sulfite iv. tricarbon octahydride vi. iron (ii) oxide c. why do certain compounds require a roman numeral? what block of elements requires them? chapter 5: soap 3. for each example below, predict if the bonding will be ionic or covalent. i. ca with cl ii. c with h iii. carbon monoxide iv. kbr v. so2 vi. magnesium iodide 4. draw lewis dot structures for these simple compounds listed below. i. br2 ii. o2 iii. n2 iv. ch4 v. nh3 vi. h2o vii. hf
Answers: 3
Do you know the correct answer?
Questions in other subjects:




French, 06.06.2020 22:00



Mathematics, 06.06.2020 22:00


English, 06.06.2020 22:00