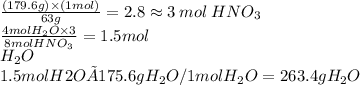Hurry NO SPAM I will mark brainliest
3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In...

Chemistry, 20.10.2021 21:50, coolman5999alt
Hurry NO SPAM I will mark brainliest
3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many moles of water can be made when 179.6 grams of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
I already know the answer, so get it right and I will give Brainliest.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1


Chemistry, 23.06.2019 08:10, 20dyeaubn
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
Do you know the correct answer?
Questions in other subjects:




Mathematics, 02.12.2020 02:00

Mathematics, 02.12.2020 02:00

English, 02.12.2020 02:00


English, 02.12.2020 02:00