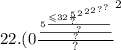Photographic film contains silver bromide in gelatin. Once exposed, some of the silver bromide decomposes, producing fine grains of silver. The unexposed silver bromide is removed by treating the film with sodium thiosulfate. Soluble sodium silver thiosulfate (Na3Ag(S2O3)2) is produced.
AgBr(s) + 2 Na2S2O3(aq) Na3Ag(S2O3)2(aq) + NaBr(aq)
Determine the mass of Na3Ag(S2O3)2 produced if 0.360 g AgBr is removed.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 02:00, sakria2002
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3

Do you know the correct answer?
Photographic film contains silver bromide in gelatin. Once exposed, some of the silver bromide decom...
Questions in other subjects:



Health, 26.05.2020 22:04


Mathematics, 26.05.2020 22:57



Mathematics, 26.05.2020 22:57

Spanish, 26.05.2020 22:57