
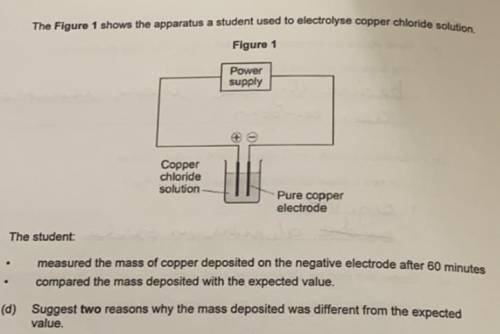
The Figure 1 shows the apparatus a student used to electrolyse copper chloride solution
The student:
- measured the mass of copper deposited on the negative electrode after 60 minutes
- compared the mass deposited with the expected value.
Suggest two reasons why the mass deposited was different from the expected
value.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3


Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
Do you know the correct answer?
The Figure 1 shows the apparatus a student used to electrolyse copper chloride solution
The studen...
Questions in other subjects:



Mathematics, 29.10.2021 14:00

Mathematics, 29.10.2021 14:00

Social Studies, 29.10.2021 14:00



Mathematics, 29.10.2021 14:00

English, 29.10.2021 14:00

SAT, 29.10.2021 14:00






