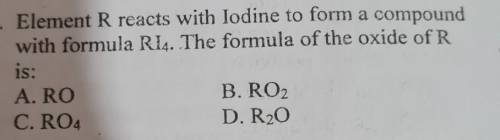Give an explanation for the answer
...

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 21.06.2019 22:30, mauifrifer3986
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1


Chemistry, 23.06.2019 07:00, jennnifercrd59jc
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Arts, 23.06.2020 20:01

History, 23.06.2020 20:01

Spanish, 23.06.2020 20:01


English, 23.06.2020 20:01