
Chemistry, 11.10.2021 18:40, donnafranks2003
An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2. After performing the experiment, 13.19 g of calcium carbonate, CaCO3, is produced. Calculate the percent yield of this reaction.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, angelteddy033
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2


Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 01:30, Thunderalesis7855
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
Do you know the correct answer?
An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2....
Questions in other subjects:


Social Studies, 04.11.2020 01:00

Geography, 04.11.2020 01:00

Mathematics, 04.11.2020 01:00


Mathematics, 04.11.2020 01:00

Mathematics, 04.11.2020 01:00


Mathematics, 04.11.2020 01:00

History, 04.11.2020 01:00


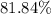 .
.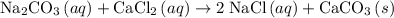 .
.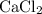 , as well as those in the product of interest,
, as well as those in the product of interest, 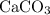 :
: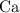 :
: 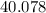 .
.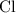 :
: 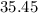 .
. :
: 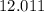 .
. :
:  .
.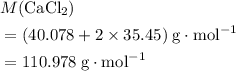 .
.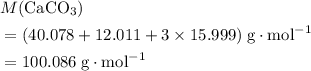 .
.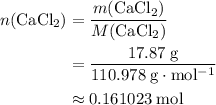 .
.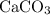 ) are both
) are both  . Thus:
. Thus: .
. 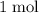 of
of 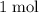 of
of 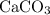 in this experiment:
in this experiment: .
. .
. , calculate the percentage yield of this experiment:
, calculate the percentage yield of this experiment: .
.



