
Chemistry, 11.10.2021 16:50, thutch1950oww9q0
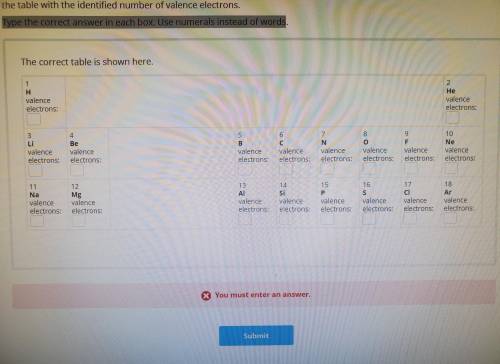
The Lithium atom has only two energy levels. The third is empty. Therefore, we can refer to the second energy level of the lithium atom as the outermost level. The electrons in this outermost energy level are called valence electrons. Refer to the table you just constructed, and determine the number of valence electrons for the first 18 elements. Fill in the table with the identified number of valence electrons Type the correct answer in each box. Use numerals instead of words.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1


Do you know the correct answer?
The Lithium atom has only two energy levels. The third is empty. Therefore, we can refer to the seco...
Questions in other subjects:

Arts, 08.06.2021 23:00


Chemistry, 08.06.2021 23:00

Social Studies, 08.06.2021 23:00



Mathematics, 08.06.2021 23:00


Mathematics, 08.06.2021 23:00

Mathematics, 08.06.2021 23:00






