
ATOMS-
1. an atom is the smallest part of a pure substance which retains the _ of that pure substance
COMPOUNDS-
2. atoms of each element are essentially the _
LAW OF DEFINITE PROPORTIONS-
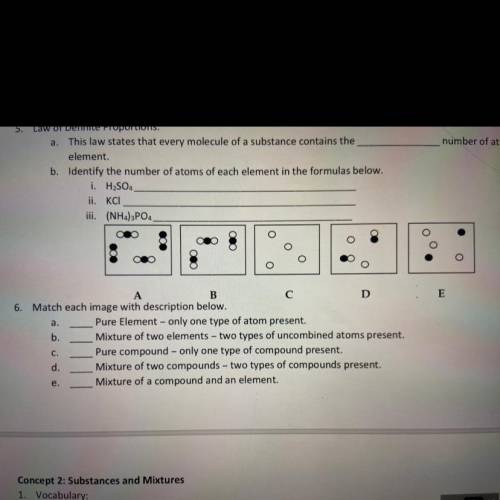
3. this law states that every molecule of a substance contains the _ number of atoms of each element
SOLUTIONS-
4. Solutions are special types of mixtures which are _ so they often can be confused as pure substances
STATES OF MATTER-
5. how would you describe the change in arrangement of particles as heat energy and temperature increases?
6. why does the temperature remain constant during a phase change even though the substance is absorbing heat energy?
7. (number six only)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
Do you know the correct answer?
ATOMS-
1. an atom is the smallest part of a pure substance which retains the _ of that pure substa...
Questions in other subjects:



Mathematics, 18.01.2021 18:20


Mathematics, 18.01.2021 18:20

English, 18.01.2021 18:20



Mathematics, 18.01.2021 18:20

Mathematics, 18.01.2021 18:20






