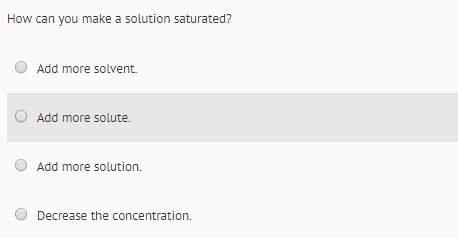
Chemistry, 11.10.2021 03:50, horsedoggal1234
BaCO3(s) → BaO(s) + CO2(g)
(a) A student heated a 10.0 g sample of barium carbonate until it was fully decomposed.
(i) Calculate the number of moles of barium carbonate the student used.
moles of barium carbonate =

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
Do you know the correct answer?
BaCO3(s) → BaO(s) + CO2(g)
(a) A student heated a 10.0 g sample of barium carbonate until it was f...
Questions in other subjects:

English, 28.11.2020 02:50




English, 28.11.2020 02:50