Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, monnicawilliam
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2


Do you know the correct answer?
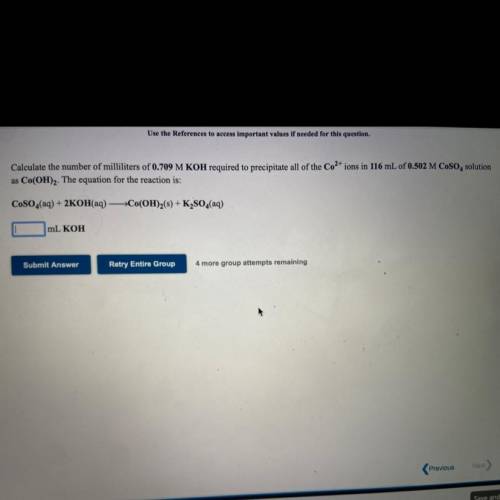
Calculate the number of milliliters of 0.709 M KOH required to precipitate all of the Co2+ ions in 1...
Questions in other subjects:



Chemistry, 26.06.2019 09:00

Mathematics, 26.06.2019 09:00


Physics, 26.06.2019 09:00

Mathematics, 26.06.2019 09:00

Chemistry, 26.06.2019 09:00

Health, 26.06.2019 09:00