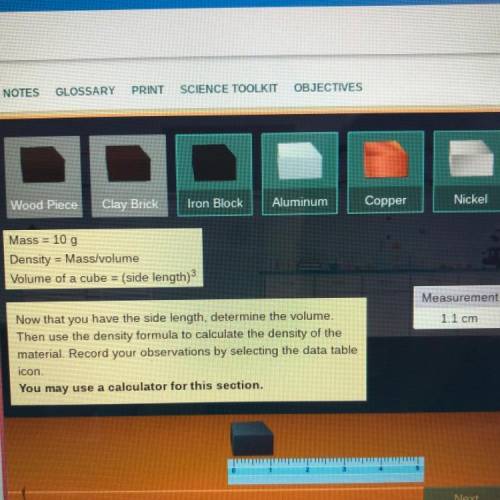
Mass = 10 g
Density = Mass/volume
Volume of a cube = (side length)3
Measurement
...

Chemistry, 09.10.2021 21:40, jaychavez1926
Mass = 10 g
Density = Mass/volume
Volume of a cube = (side length)3
Measurement
1.1 cm
Now that you have the side length, determine the volume.
Then use the density formula to calculate the density of the
material. Record your observations by selecting the data table
icon.
You may use a calculator for this section.
Measurement
1.1 cm
Please help me explain to me how you can determine the Volume I don’t know how to do that and I need help ASAP today is the last day to do this and I really need help

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Physics, 26.10.2019 22:43

Mathematics, 26.10.2019 22:43




Mathematics, 26.10.2019 22:43


Social Studies, 26.10.2019 22:43


Social Studies, 26.10.2019 22:43






