
Use the image attached to answer the following questions ASAP please!!
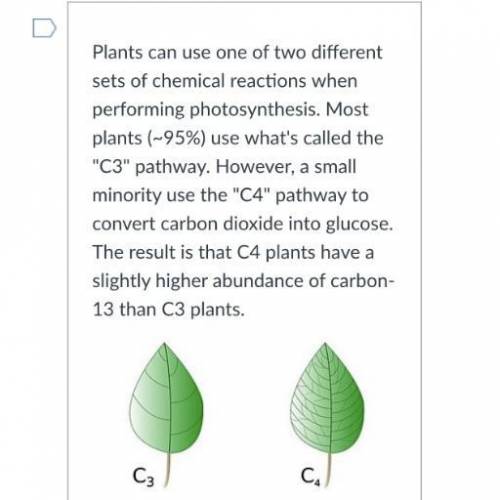
⍣Honey (made from C3 plant sources) has the following C - 12 / C - 13 abundances. Calculate the average atomic mass of carbon in honey -12:98.934896 -131.0652 %
⍣ How much would 42, 000, 0m moles of glucose (C_{6}*H_{12} * O_{6}) extracted from honey weigh in grams? (Assume oxygen and hydrogen have their normal atomic mass.)
⍣ A 1.238756 mole sample of sugar from a jelly sample weighs 223,1716910 g this jelly most likely sweetened with sugar sugar cane or with honey ? Explain your reasoning.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, gallegosarmanni
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3
Do you know the correct answer?
Use the image attached to answer the following questions ASAP please!!
⍣Honey (made from C3 plant...
Questions in other subjects:

Mathematics, 18.02.2020 19:43



Mathematics, 18.02.2020 19:44











