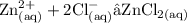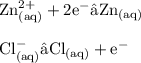Using the reaction of Zinc with dilute hydrochloric acid:
a) Write an ionic equation for the reaction, with state symbols. (3 marks)
b) From your answer to part a construct two half equations to show electron transfer taking place.(2 marks)
c) Explain why this is a redox reactor (5 marks)

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
Do you know the correct answer?
Using the reaction of Zinc with dilute hydrochloric acid:
a) Write an ionic equation for the react...
Questions in other subjects:


Mathematics, 23.11.2020 20:50


Mathematics, 23.11.2020 20:50

Mathematics, 23.11.2020 20:50


Mathematics, 23.11.2020 20:50


Mathematics, 23.11.2020 20:50

Chemistry, 23.11.2020 20:50