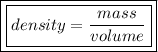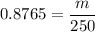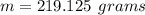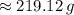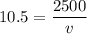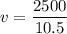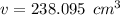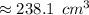Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, 2019reynolds
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 13:00, nadikadiaz1
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 23.06.2019 05:30, jalynholden07
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 09:00, payshencec21
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
Do you know the correct answer?
50 Points Help
1) Find the mass of 250.0 mL of benzene. The density of benzene is 0.8765 g/mL.
Questions in other subjects:


History, 08.12.2021 17:50

History, 08.12.2021 17:50

Mathematics, 08.12.2021 17:50




Mathematics, 08.12.2021 17:50


English, 08.12.2021 17:50