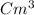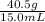Chemistry, 04.10.2021 20:30, Victoriag2626
50 Points Help
1) A block of aluminum occupies a volume of 15.0 mL and mass 40.5 g. What is its density?
2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder mass 306.0 g. From this information, calculate the density of mercury.
3) What is the mass of the ethyl alcohol that exactly fills a 200.0 mL container? The density of ethyl alcohol is 0.789 g/mL.
4) A rectangular block of copper metal weighs 1896 g. The dimensions of the block are 8.4 cm by 5.5 cm by 4.6 cm. From this data, what is the density of copper? ( hint find the volume first: volume of rectangle = Hight x width x length)
5) Calculate the density of sulfuric acid if 35.4 mL of the acid mass 65.14 g.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, lilque6112
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
Do you know the correct answer?
50 Points Help
1) A block of aluminum occupies a volume of 15.0 mL and mass 40.5 g. What is its de...
Questions in other subjects:

Social Studies, 18.08.2020 22:01









Computers and Technology, 18.08.2020 22:01


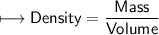

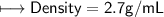

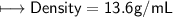

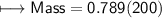
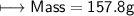
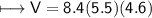
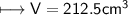
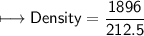
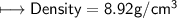
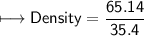
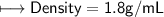
 when dealing with a solid.
when dealing with a solid.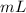 or
or