
Chemistry, 04.10.2021 14:00, daniel1480
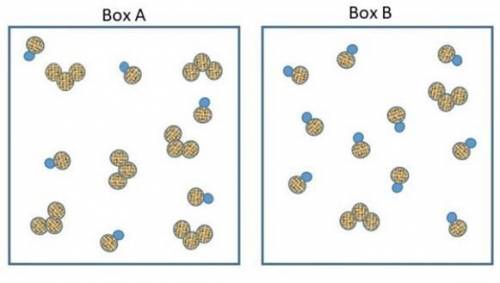
The two pictures shown below represent starting conditions for the following reaction: O3 (g) + NO(g) → O2 (g) + NO2 (g) with a rate law: Rate = k (O3 )(NO)
Which flask will react faster than the other? Determine how much faster the fast one reacts compared to the slow one? Explain your answers in terms of molecular collisions.
How do I find the answer to this? My thinking is that box a will react more since there's equal amounts of reactants but box b will react faster since there's so much NO to collide with the O3 molecules. Thanks.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
Do you know the correct answer?
The two pictures shown below represent starting conditions for the following reaction: O3 (g) + NO(g...
Questions in other subjects:

History, 12.01.2021 01:50


Geography, 12.01.2021 01:50







Chemistry, 12.01.2021 01:50






