
Chemistry, 02.10.2021 22:20, notsobright10
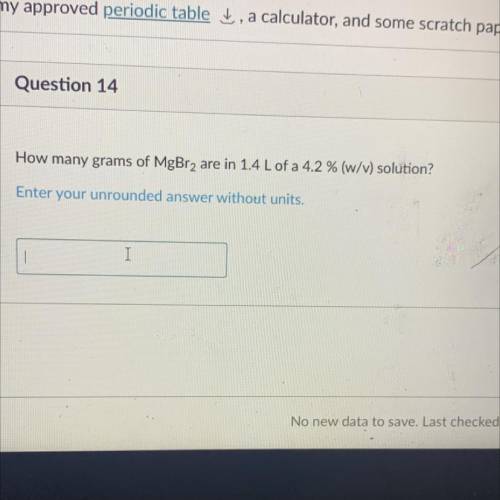
How many grams of MgBr2 are in 1.4 Lof a 4.2% (W/M) solution?
Enter your unrounded answer without units,
I

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
Do you know the correct answer?
How many grams of MgBr2 are in 1.4 Lof a 4.2% (W/M) solution?
Enter your unrounded answer without...
Questions in other subjects:

Chemistry, 12.02.2021 03:30

Chemistry, 12.02.2021 03:30


English, 12.02.2021 03:30

English, 12.02.2021 03:30


Social Studies, 12.02.2021 03:30

Mathematics, 12.02.2021 03:30

Mathematics, 12.02.2021 03:30







