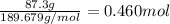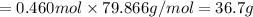Chemistry, 29.09.2021 21:20, Rflaig1129841
When TiCl4 (s) reacts with H20 (), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g of
titanium (IV) chloride present, with water in excess, how much solid titanium (IV) oxide (in grams)
could theoretically be produced?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2


Chemistry, 23.06.2019 10:30, oglejack6138
How is it possible for someone to put an ear to a wall and hear someone in the next room? a. sound waves can travel though solids. b. the waves travel from room to room via air. c. there must be some air in the wall so the sound can travel through it. d. sound waves change to electromagnetic waves and then back again.
Answers: 1
Do you know the correct answer?
When TiCl4 (s) reacts with H20 (), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g...
Questions in other subjects:

History, 21.08.2019 15:30

History, 21.08.2019 15:30

Physics, 21.08.2019 15:30


Mathematics, 21.08.2019 15:30

History, 21.08.2019 15:30

Social Studies, 21.08.2019 15:30

English, 21.08.2019 15:30