

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1



Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
Do you know the correct answer?
A beaker contains 1.00 x 102 grams of 0.700 M NaCl. If you transfer 50.0 grams of the solution to an...
Questions in other subjects:


World Languages, 22.06.2021 14:00

Computers and Technology, 22.06.2021 14:00

Geography, 22.06.2021 14:00

Arts, 22.06.2021 14:00


English, 22.06.2021 14:00

Biology, 22.06.2021 14:00

Computers and Technology, 22.06.2021 14:00


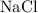 solution in the first beaker would continue to be
solution in the first beaker would continue to be  .
.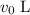 , and that
, and that  of the solution was transferred to the other beaker. (Because of the
of the solution was transferred to the other beaker. (Because of the  might not necessarily be
might not necessarily be 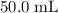 .) In this question,
.) In this question, 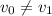 .
.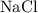 in that solution:
in that solution: .
.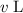 of transferred solution would be
of transferred solution would be 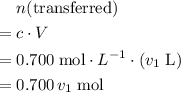 .
. .
.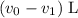 .
.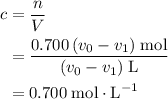 .
.



