Electrochemistry is important in many aspects of daily life.
i. Define voltaic cell.
ii. Fi...

Chemistry, 26.09.2021 17:10, isabellemdeakin
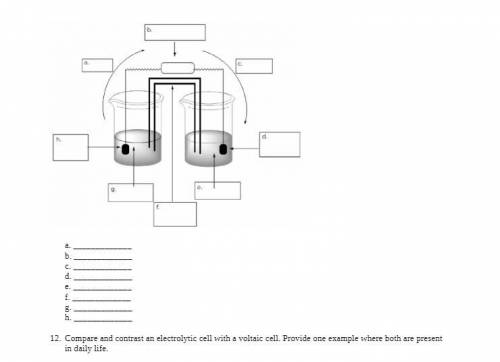
Electrochemistry is important in many aspects of daily life.
i. Define voltaic cell.
ii. Fill in the blanks for the drawing of a voltaic cell that’s made with copper/copper (II) nitrate (E° =
0.34 V) and zinc/zinc (II) nitrate (E° = –0.76 V). Briefly explain the role of the salt bridge.
iii. Using the equation E°cell = E°cathode – E°anode, calculate the overall cell potential for the cell in step b.
Be sure to show all steps completed to arrive at the answer

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, johnnydenali67
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1


Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
Do you know the correct answer?
Questions in other subjects:




Mathematics, 01.01.2022 15:30


Mathematics, 01.01.2022 15:30


English, 01.01.2022 15:30


Chemistry, 01.01.2022 15:30






