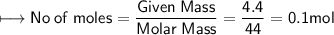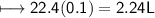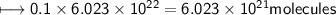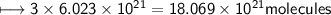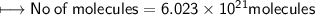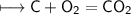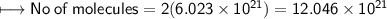Chemistry, 26.09.2021 05:00, nidiavega2009
A closed cylinder is filled with CO₂ gas. The mass of CO₂ in cylinder is 4.4 g. Now express this amount of Carbon dioxide in following terms:
a) no. of moles of CO₂ molecules
b) Volume at NTP
c) No. of 'gram molecule
d) No. of CO₂ molecules
e) No. of carbon atoms
f) No. of mole of Oxygen atoms
g) No. of molecules of oxygen

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2
Do you know the correct answer?
A closed cylinder is filled with CO₂ gas. The mass of CO₂ in cylinder is 4.4 g. Now express this amo...
Questions in other subjects:


Mathematics, 07.12.2020 23:50


Arts, 07.12.2020 23:50


History, 07.12.2020 23:50