Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, dice50
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 01:30, thickness7699
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1


Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
Do you know the correct answer?
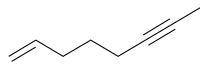
The name of the structure shown is
Question 17 options:
A)
oct-1-ene-6-yn...
A)
oct-1-ene-6-yn...
Questions in other subjects:



English, 02.08.2019 22:00

History, 02.08.2019 22:00



Mathematics, 02.08.2019 22:00