
Chemistry, 22.09.2021 02:30, gwendallinesikes
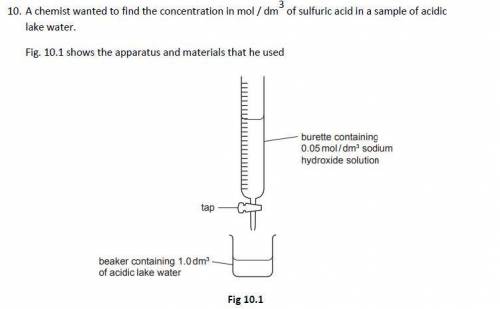
The chemist slowly added 0.05 mol / dm3 sodium hydroxide solution to 1.0 dm3 of acidic lake water contained in a beaker until the acid had just been neutralised
The chemist found that it required 12.5 cm3 of 0.05 mol / dm3 sodium hydroxide solution to neutralise the acid
a. State the number of moles of sodium hydroxide which are dissolved in 1.0 dm3 of the sodium hydroxide solution
b. Calculate the number of moles of sodium hydroxide which are dissolved in 12.5 cm3 of the sodium hydroxide solution. Show your workings.
Show your working.
c. The balanced equation for the neutralisation reaction is
2NaOH + H2SO4 → Na2SO4 + 2H2O
Calculate the number of moles of sulfuric acid which were contained in 1.0 dm3 of acidic lake water.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
Do you know the correct answer?
The chemist slowly added 0.05 mol / dm3 sodium hydroxide solution to 1.0 dm3 of acidic lake water co...
Questions in other subjects:



Mathematics, 27.09.2020 20:01

Mathematics, 27.09.2020 20:01




Law, 27.09.2020 20:01








