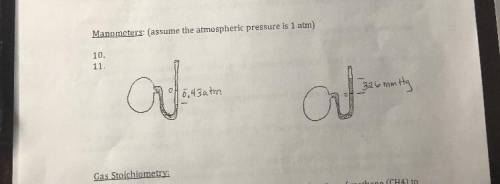Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 23.06.2019 03:30, alecnewman2002
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
Do you know the correct answer?
Worth 50 points (25 each)
I am honestly not even sure what we are supposed to be solving for! An e...
Questions in other subjects:

Mathematics, 04.02.2021 17:30


History, 04.02.2021 17:30

Mathematics, 04.02.2021 17:30

Mathematics, 04.02.2021 17:30

Spanish, 04.02.2021 17:30



SAT, 04.02.2021 17:40