
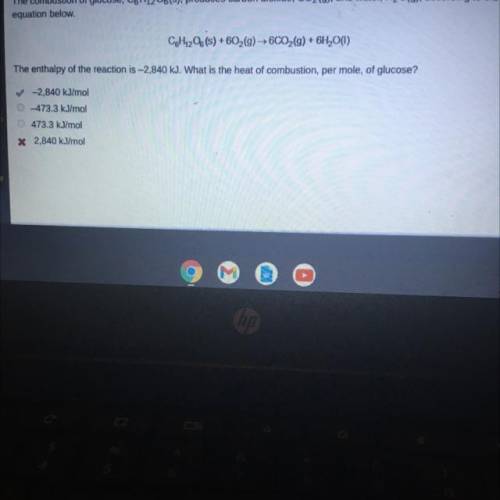
The combustion of glucose, C6H1206(s), produces carbon dioxide, CO2(g), and water, H2O(g), according to the
equation below.
CoH1206(s) +602(g) → 6CO2(g) + 6H2O(1)
The enthalpy of the reaction is -2,840 kJ. What is the heat of combustion, per mole, of glucose?
✓-2,840 kJ/mol
473.3 kJ/mol
473.3 kJ/mol
* 2,840 kJ/mol
See

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, creepycrepes
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
Do you know the correct answer?
The combustion of glucose, C6H1206(s), produces carbon dioxide, CO2(g), and water, H2O(g), according...
Questions in other subjects:



Mathematics, 09.03.2021 22:30

Mathematics, 09.03.2021 22:30


Mathematics, 09.03.2021 22:30

English, 09.03.2021 22:30



Mathematics, 09.03.2021 22:30






